(continued from Little Things (i))
Very little and insignificant things have indeed the ability to change our lives. But there is still another quite little thing that, in fact, keeps us alive.
Everyone knows H2O as the chemical formula of water. A chemical formula is a convenient way to count how many atoms of each element are present in a given chemical compound. H2O means that a single water molecule is composed by two hydrogen atoms and one oxygen atom. Now oxygen has two free bonds (valence 2) and hydrogen has one free bond (valence 1). An oxygen atom with its two bonds taken each by a hydrogen atom forms a water molecule.
We can imagine each one of the atoms as a tiny ball, and a chemical bond as an even tinier stick that keeps two of the balls together. Now the oxygen atom is a slightly bigger ball in the middle with two sticks to the sides, each one connected to a hydrogen atom. These two bonds at the sides of the oxygen atom are not perfectly aligned, which would mean a 180° angle, but instead they form a slightly obtuse angle (exactly at 104.45°). Apparently an insignificant fact. But only apparently.
Oxygen has a higher electronegativity (3.44) than hydrogen (2.20), meaning that an oxygen atom attracts electrons much stronger than a hydrogen atom does.
The 104.45° angle between the two oxygen bonds means that one side of the water molecule (the oxygen's side) attracts electrons stronger than the other side (hydrogen atoms' side). We say water molecule has a dipolar nature. A water molecule is, in a way, like a tiny magnet, which has a positive and a negative pole.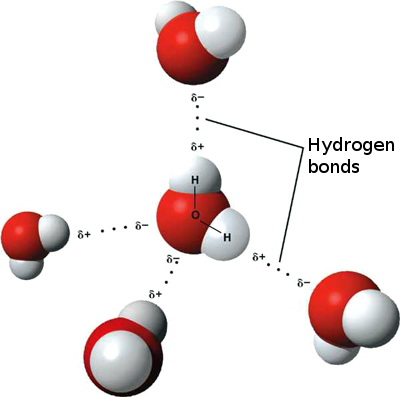
(Image taken from Wikimedia Commons)
What happens is that the oxygen side (the negative pole) attracts all electrons in the vicinity, including those electrons in the hydrogen atoms of neighbouring water molecules. That means water molecules are bonded together through its dipolar nature. This kind of bonding is called a hydrogen bond, and it is weaker than a normal (covalent) bond, like the one that keeps a single molecule together. Still, hydrogen bonding among water molecules is responsible for the fact that water is liquid at room temperature, because more heat energy is required to break the hydrogen bonds between molecules.
Now take a moment to think about it. If oxygen had its two free bonds at a 180° angle, there would be no positive and negative side in the water molecule, because the two hydrogen atoms would cancel out the higher electron affinity of oxygen. The boiling point would be much much lower than 100°C. In fact, without hydrogen bonding, water would be a gas at room temperature.
Now imagine all oxygen atoms aligned its bonds at once. That means almost all liquid water on our planet would simply evaporate. Life as we know it would simply cease to exist. At once...
Thank goodness there is that 104.45° angle!
Saturday, 7 March 2009
Little Things (ii)
Subscribe to:
Post Comments (Atom)

 versión en español
versión en español






4 comments:
Oxygen hydrogen mix----what a miracel ,for me it is a wonderful sea..........LG.G.
Gitta, it is certainly a miracle! I could spend hours and hours just looking into the sea...
Übrigens, es ist für mich überhaupt kein Problem, wenn Du weiter Deine Kommentare auf Deutsch schreibst! Ich werde sie übersetzen und die Antworten auf Englisch schreiben. Es ist mir sehr wichtig, dass liebe Leute wie Du weiter in meinem Blog kommentieren. Und wenn dann, auf die einfachste Weise, in der wir kommunizieren können. In unserem Fall also, auf Deutsch!
Viele liebe Grüsse,
Toni :)
Ich versteh Deine Sehnsucht nach der See,Ich hatte Jahre lang Sehnsucht (Heimweh) nach einem knorrigen alten Kirschbaum:-)LG.G
Gitta said...
I understand your longing for the sea. I longed years and years for an old knotty cherry tree :-)
Gitta, interesting... a cherry tree... Were you in a land with no cherry trees?
Post a Comment