I already told you about my struggle on growing Mediterranean tree species in Austria. Well, our orange trees are doing pretty well, but I am starting to loose my faith on the survival of the pine tree. I think it might have frozen as we were in Spain last Christmas.
I'm afraid the day will come when I'll have to accept the evidence and throw my little pine away. Knowing that I will be very sad about it, I decided to make up to myself for it by letting new life sprout before the day comes. Just in case.
It all started in the Paleoproterozoic, around 3000 million years ago, as the ice cap of the Makganyene glaciation (which might have covered the whole planet) retreated and left space for a vast ancestral ocean.
Two types of living organisms survived under the ice and rapidly populated this ocean. Archaea, which used several sources of energy such as sulphur or ammonia and produced methane as waste product, and a new kind of bacteria, the Cyanobacteria, able to obtain their energy through photosynthesis, a process that converts sunlight and carbon dioxide (CO2) into energy, releasing oxygen (O2) as a waste product.
These cyanobacteria were so successful at reproducing and colonising the ocean that they actually changed the composition of the atmosphere and were going to shape the world for the next 3000 million years. Before cyanobacteria existed, there was not much oxygen in the atmosphere. Free oxygen in large amounts was in fact poisonous to most of the living creatures at the time, especially to archaea. Most life on Earth vanished in what is known as the Oxygen Catastrophe(*).
But serious stress factors like this great oxidizing event are notably the source of big evolutionary pushes: a whole new kind of bacterial organisms evolved, aerobic bacteria, which used oxygen (now available in huge amounts) as a source of energy, producing carbon dioxide as a waste product. And that's not all. By an outstanding touch of genius, some Archaea, facing a serious problem in this new and poisonous oxygen-rich environment, incorporated cyanobacteria or proteobacteria (a kind of aerobic bacteria) through the cellular membrane into their cellular bodies and used part of the energy produced by them to keep themselves alive. This brilliant trick, known as endosymbiosis, would be the origin of the eukaryotic cells, which contain several membrane-bound structures, each one specialising in a different function.
Those Archaea that associated with aerobic bacteria (which needed oxygen) were the basis for the fauna, to which we humans (**) belong as well. From Archaea that associated with cyanobacteria (which produced oxygen through photosynthesis) would originate the flora, that is, multi-celled living creatures able to obtain energy from sunlight through photosynthesis. (***)
(***)
I've been thinking about this long story, this long evolutive path, as I was sowing orange and lemon seeds to give plant life another chance. Now wake up!, you little seeds. Wake up from your long sleep, let sunshine and water put you in alert and consummate once again the miracle of life for me!
(*) This Oxygen Catastrophe in the Paleoproterozoic raised the atmospherical oxygen concentration to 4%. To reach the current level of 21% a colonisation of the land masses by plants and woods would be needed.
(**) Not all Archaea disappeared in the Paleoproterozoic. In fact, a great number of them are alive and well, living in the most incredible places. Big colonies of Methanobrevibacter smithii, for instance, live in the human gut and help us with digestion.
(***) Taronger is the Catalan word for "orange tree".
Sunday, 29 March 2009
Hoping for new life
Tuesday, 17 March 2009
Blogger of the month! :)
Die Murmeltierjahre im Land des Frühschoppens has been chosen Blog of the Month for March 2009 by Expat blog, the expatriate community.
You can read the interview here. Thanks Olivier, Julien and Benjamin! :)

Monday, 16 March 2009
Saturday, 7 March 2009
Little Things (ii)
(continued from Little Things (i))
Very little and insignificant things have indeed the ability to change our lives. But there is still another quite little thing that, in fact, keeps us alive.
Everyone knows H2O as the chemical formula of water. A chemical formula is a convenient way to count how many atoms of each element are present in a given chemical compound. H2O means that a single water molecule is composed by two hydrogen atoms and one oxygen atom. Now oxygen has two free bonds (valence 2) and hydrogen has one free bond (valence 1). An oxygen atom with its two bonds taken each by a hydrogen atom forms a water molecule.
We can imagine each one of the atoms as a tiny ball, and a chemical bond as an even tinier stick that keeps two of the balls together. Now the oxygen atom is a slightly bigger ball in the middle with two sticks to the sides, each one connected to a hydrogen atom. These two bonds at the sides of the oxygen atom are not perfectly aligned, which would mean a 180° angle, but instead they form a slightly obtuse angle (exactly at 104.45°). Apparently an insignificant fact. But only apparently.
Oxygen has a higher electronegativity (3.44) than hydrogen (2.20), meaning that an oxygen atom attracts electrons much stronger than a hydrogen atom does.
The 104.45° angle between the two oxygen bonds means that one side of the water molecule (the oxygen's side) attracts electrons stronger than the other side (hydrogen atoms' side). We say water molecule has a dipolar nature. A water molecule is, in a way, like a tiny magnet, which has a positive and a negative pole.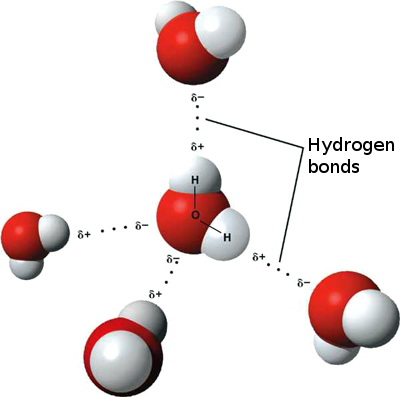
(Image taken from Wikimedia Commons)
What happens is that the oxygen side (the negative pole) attracts all electrons in the vicinity, including those electrons in the hydrogen atoms of neighbouring water molecules. That means water molecules are bonded together through its dipolar nature. This kind of bonding is called a hydrogen bond, and it is weaker than a normal (covalent) bond, like the one that keeps a single molecule together. Still, hydrogen bonding among water molecules is responsible for the fact that water is liquid at room temperature, because more heat energy is required to break the hydrogen bonds between molecules.
Now take a moment to think about it. If oxygen had its two free bonds at a 180° angle, there would be no positive and negative side in the water molecule, because the two hydrogen atoms would cancel out the higher electron affinity of oxygen. The boiling point would be much much lower than 100°C. In fact, without hydrogen bonding, water would be a gas at room temperature.
Now imagine all oxygen atoms aligned its bonds at once. That means almost all liquid water on our planet would simply evaporate. Life as we know it would simply cease to exist. At once...
Thank goodness there is that 104.45° angle!


 versión en español
versión en español